

Whisky, flavours, and headspace concentrations
Recently, two scientific articles of Conner et al. on whisky caught my attention, namely, Headspace concentrations of ethyl esters at different alcoholic strengths and Release of distillate flavour compounds in Scotch malt whisky (1, 2).
These articles sound rather complicated and are they really of interest for the non-scientific whisky enthusiast?
If you are interested in knowing what is happening to your whisky after you have opened your bottle or what is the impact of the bottling strength on the taste of your whisky, then keep on reading this report.
In the first publication, Conner et al. is looking at the concentration of the ethyl esters at different strength.
Introduction:
“ethyl esters” and “headspace”?
Ethyl ester
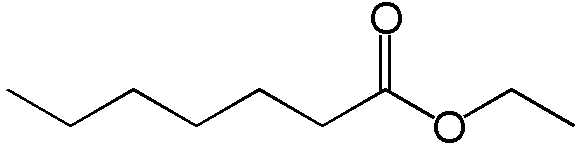
Whisky, from a chemical perspective, is a very dirty product. The main components are water and ethanol (alcohol, generally 40-43% of the mixture)). However, drinking pure alcohol is rather tasteless and whisky is rich in flavour. The flavours of the whisky are coming from different chemical compounds generated during the process of transforming the barley in new make spirits, as well as during the maturation, when the distillate is interacting with the wood of the casks. Esters are key flavour-active molecule in whisky and contributes to flavours such as pineapple, strawberries, apples or apricots, but also to less pleasant flavours (eg. soapy). An example of an ethyl ester (EE) chemical structure (Ethyl heptanoate) is illustrated below. Basically, it is a an assembly of atoms of carbon bound with two molecules oxygen (the “o”) close to each other.
Headspace
The headspace is the volume of air above the surface of the liquid (e.g., whisky). In a whisky bottle, the headspace is the volume between the whisky and its cork/capsule
The experience
To investigate the effect of the concentration of the alcohol on the headspace concentration of ethyl esters, they filled glass vials with a mixture of water, ethanol (alcohol) at different concentrations (between 5 and 80 % alcohol by volume (ABV) and a combination of different ethyl esters (mixture of EE with different acid methylene groups (-CH3)). At a certain time, with a syringe, they withdrew some air in the headspace and injected this air in an analytical device (gas chromatograph) to determine the concentration of different volatile EE. These concentrations in headspace are expressed in “activity coefficient”.
The results
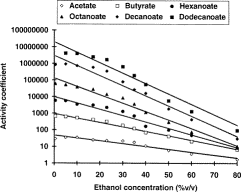
Fig. 1: Effects of increasing ethanol concentrations of the activity coefficient of ethyl esters. Copied from (1).
On figure 1, we can see that the activity coefficient (AC) increases when the ethanol concentration decreases. This increase in the AC is more pronounced for the small molecules (e.g., acetate and butyrate) than for the large ones (e.g., decanoate and dodecanoate). This increase is however stronger on the left side of the x axis than on the right side.
What does it mean?
When you translate the figure 1 in simpler terms, it means that if you reduce the ethanol volume from 15 % (e.g., wine) to 5% (beer), this would have little influence on the flavours and the taste of your alcoholic beverage. However, if you would reduce the strength of your whisky bottle from 40% to 30-35%, the activity coefficient would increase considerably. In over terms, the pleasant flavours of the whisky would move from the liquid into the air between the whisky and the cork, and after you have opened your bottle, it would loose quickly its characteristics. Even more important is the fact that the small molecule would move faster to the headspace than the large one. This is quite annoying, since the small EE are producing the nice flavours and the large ones, the unpleasant one (soap-like).
In conclusion, based on this study, when your whisky is decreasing in alcoholic strength, it will loose first its more pleasant flavours and the soap-like ones would be more pronounced.
Several whisky enthusiasts, once they have opened and drank a portion of their bottle, are transferring the whisky in smaller bottles and are claiming that it improves the quality of the stored whisky.
In this experiment, the headspace volume in the vials was quite large (about the same volume as the ethanol mixture volume) and by reducing the volume of headspace, we might expect to reach saturation in the headspace and thus retain the whisky flavours, therefore, transferring the whisky into smaller bottles appears to be a good solution for maintaining the flavours of your whisky. However, I suspect that these volatile esters will remain partially in suspension in the headspace, between the liquid and the cork and transferring too often your whisky into smaller bottle might have the same detrimental effect as keeping a large bottle almost empty.
In the second publication, Conner et al. investigated the effect of the temperature and wood extracts of on the activity coefficient. When the temperature increased from 20 to 25°C, the effects on the activity coefficient of ethyl esters were slightly affected, but more pronounced when temperature is increased to 30°C. Interestingly, when introducing oak chips to the mixture, the headspace concentration of ethyl esters was markedly reduced (see figure 2). This would indicate that the wood extract or cask particles would improve the stability of the ethanol/water mixture.
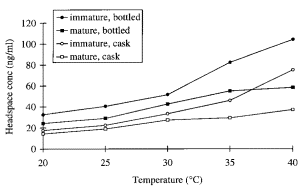
Fig. 2: Ethyl decanoate headspace concentrations over model bottled and cask malt whiskies diluted to 23% ethanol (v/v) at different temperatures. Copied from (2).
General conclusion and discussion
By law, whisky should be at least 40% ABV. Since duties on whisky are high, whisky companies traditionally bottle their whiskies at 40-43% in order to maintain the price of a bottle of whisky affordable. Nowadays, more and more whisky connoisseurs are interested in cask strength whiskies and non-chill-filtrated. During chill-filtration, a process that allows the precipitation of some fatty acids, which would otherwise contribute to a cloudy (“haze”) in a whisky reduced to 40-43%.
The work of Conner et al. is rather interesting. In models of whisky, they have tried to investigate the effect of different variables (ethanol concentrations, temperature and wood extracts) on the flavours of whisky (ethyl esters). The conclusions were that, by decreasing the ethanol (alcohol) concentration to below 40% ABV was detrimental to the whisky flavours, that temperature had only slight effects, and the wood extracts would increase the stability of the whisky.
In other words, if the model used by Conner et al., can be considered as representative for a bottle of whisky, the results that they have obtained suggest that whiskies bottled at 40-43%, once opened, would loose their flavours faster than a whisky bottled at 46%. In addition, with time, the proportion of unpleasant flavours vs pleasant flavours would increase. Furthermore, by filtrating the wood particles from the whisky, the stabilizing effects of the wood extracts on the whisky flavours would be removed. In conclusion, a whisky bottled at cask strength and non-filtrated would hold the effect of time better than a whisky bottled at 40%. Whisky is a more complex mixture than the model used by Conner et al., and since the temporal relationship with the reduction of flavours is not established in these publications, just enjoy the whisky with moderation, but it could be advisable to finish your opened bottles before starting a new one, in order to retain the maximum of taste sensations.
1. J. M. Conner, L. Birkmyre, A. Paterson, J. R. Piggott, Journal of the Science of Food and Agriculture 77, 121 (1998).
2. J. M. Conner, A. Paterson, J. R. Piggott, Journal of the Science of Food and Agriculture 79, 1015 (1999).
Dr P. Brossard, May 2008